MDISS INITIATIVES
Helping partners develop practical solutions for device security and patient safety
- All
- DATA ACQUISITION
- QUALITY & ANALYTICS
- PRACTICES & OUTCOMES
- ADVOCACY & EDUCATION
- COMMUNITY

MDRAP™
MDRAP™ (medical device risk-assessment platform) is a cyber risk assessment and data sharing platform. Results are dynamic and easy to collate. Crowdsourced from vetted Healthcare Technology professionals, MDRAP™ generates a new kind of medical device security profile – one that is easy to complete, clear, concise, and (most importantly) actionable. It’s transparent, actionable and fast – and the network effects of “crowdsourcing” mean that your team spends less time entering data and more time addressing controls.

UNIVERSITY ALLIANCES
MDISS partners with major Universities and academicians around the country to connect researchers to their counterparts on the front lines of business and healthcare. University faculty and students get special discounts on MDISS programs and memberships, and MDISS member companies benefit from personal introductions to relevant scientists and researchers.
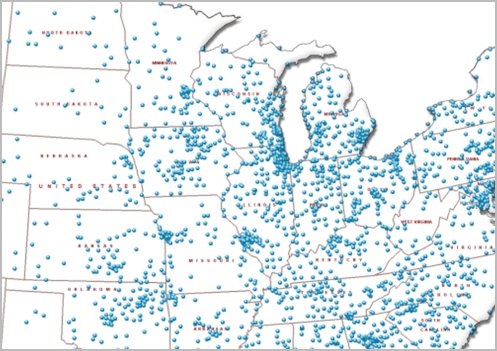
CYBER VULNERABILITY SHARING NETWORK
A pioneering initiative across more than 1,000 partner hospitals to expedite high-quality timely information sharing for medical device cyber vulnerabilities.

MEDICAL DEVICE SECURITY ASSESSMENT
The crowd sourced and expert-vetted device security evaluation and reporting platform from MDISS. MDRAP catalogs risk profiles and real-world performance data for thousands of different medical devices in situations around the world.

WHISTL™
The new MDISS World Health Information Security Testing Lab (WHISTL™) facilities will comprise of a federated network of medical device security testing labs, independently owned and operated by MDISS-member organizations. The goal is to help organizations work together to more effectively address the public health challenges arising from cyber security issues emergent in complex, multi vendor networks of medical devices. MDISS members get preferred access to WHISTL™ labs all over the world.

MD-VIPER
MD-VIPER was created through an operational partnership & MOU between the FDA, NH-ISAC and MDISS. MD-VIPER helps healthcare providers improve their situational awareness of medical device threats, as well as collect best practices and mitigation strategies from around the country.

DATA COMMONS
Consider Data Commons your secure data-sharing clearinghouse where you can share finished device risk assessments and find already-finished assessments from thousands of manufacturers, researchers, clinical engineers and other hospital stakeholders. Share best-practices and keep up with the latest vulnerabilities here, too – all while protecting your Intellectual Property and patient privacy.

AAMI + MDISS COOPERATION
MDISS participates on the working committee producing the ANSI/AAMI/IEC 80001-1 standard, specifically with regards to the application of risk management for IT Networks incorporating medical devices. It defines responsibilities for device manufacturers, non-medical device manufacturers, providers, IT integrators, and anyone else engaged in installing, using, reconfiguring, maintaining and decommissioning networks incorporating medical devices. Importantly, this standard specifically addresses risks to patients, among others.

MDISS COMMUNICATIONS PROGRAMS
Helps partners, from hospitals to device manufacturers to security firms, communicate more effectively and productively. MDISS supports member organizations that might have historically “sat” on potential problems to instead systematically evaluate and remediate the problems.

MDISS STATE-LEVEL INITIATIVES
MDISS works directly with State and Local governments to advance medical device security initiatives leveraging existing, traditional public health best practices they already understand.

MEDICALIZING IEC 62443-4
IEC 62443-4 is the international security best practices standard for vendors of industrial control systems with clear utility for medical device networks. The ISA99 Committee named MDISS as the official liaison to IEC 62443-4 responsible for “medicalizing” the standard.

MDISS ADVISORY BOARD
This is a different kind of MDISS participation; a hand-picked board of experts from across the wide swath of disciplines our work touches. The advisory board provides more long-term, strategic advice to MDISS leadership, while the Leadership Circle (above) is generally most concerned with activities that fall into a more tactical twelve-month time window.

MDRAP™
MDRAP™ (medical device risk-assessment platform) is a cyber risk assessment and data sharing platform. Results are dynamic and easy to collate. Crowdsourced from vetted Healthcare Technology professionals, MDRAP™ generates a new kind of medical device security profile – one that is easy to complete, clear, concise, and (most importantly) actionable. It’s transparent, actionable and fast – and the network effects of “crowdsourcing” mean that your team spends less time entering data and more time addressing controls.

UNIVERSITY ALLIANCES
MDISS partners with major Universities and academicians around the country to connect researchers to their counterparts on the front lines of business and healthcare. University faculty and students get special discounts on MDISS programs and memberships, and MDISS member companies benefit from personal introductions to relevant scientists and researchers.

CYBER VULNERABILITY SHARING NETWORK
A pioneering initiative across more than 1,000 partner hospitals to expedite high-quality timely information sharing for medical device cyber vulnerabilities.

MEDICAL DEVICE SECURITY ASSESSMENT
The crowd sourced and expert-vetted device security evaluation and reporting platform from MDISS. MDRAP catalogs risk profiles and real-world performance data for thousands of different medical devices in situations around the world.

WHISTL™
The new MDISS World Health Information Security Testing Lab (WHISTL™) facilities will comprise of a federated network of medical device security testing labs, independently owned and operated by MDISS-member organizations. The goal is to help organizations work together to more effectively address the public health challenges arising from cyber security issues emergent in complex, multi vendor networks of medical devices. MDISS members get preferred access to WHISTL™ labs all over the world.

MD-VIPER
MD-VIPER was created through an operational partnership & MOU between the FDA, NH-ISAC and MDISS. MD-VIPER helps healthcare providers improve their situational awareness of medical device threats, as well as collect best practices and mitigation strategies from around the country.

DATA COMMONS
Consider Data Commons your secure data-sharing clearinghouse where you can share finished device risk assessments and find already-finished assessments from thousands of manufacturers, researchers, clinical engineers and other hospital stakeholders. Share best-practices and keep up with the latest vulnerabilities here, too – all while protecting your Intellectual Property and patient privacy.

MDISS STATE-LEVEL INITIATIVES
MDISS works directly with State and Local governments to advance medical device security initiatives leveraging existing, traditional public health best practices they already understand.

MEDICALIZING IEC 62443-4
IEC 62443-4 is the international security best practices standard for vendors of industrial control systems with clear utility for medical device networks. The ISA99 Committee named MDISS as the official liaison to IEC 62443-4 responsible for “medicalizing” the standard.

AAMI + MDISS COOPERATION
MDISS participates on the working committee producing the ANSI/AAMI/IEC 80001-1 standard, specifically with regards to the application of risk management for IT Networks incorporating medical devices. It defines responsibilities for device manufacturers, non-medical device manufacturers, providers, IT integrators, and anyone else engaged in installing, using, reconfiguring, maintaining and decommissioning networks incorporating medical devices. Importantly, this standard specifically addresses risks to patients, among others.

MDISS COMMUNICATIONS PROGRAMS
Helps partners, from hospitals to device manufacturers to security firms, communicate more effectively and productively. MDISS supports member organizations that might have historically “sat” on potential problems to instead systematically evaluate and remediate the problems.

MDISS ADVISORY BOARD
This is a different kind of MDISS participation; a hand-picked board of experts from across the wide swath of disciplines our work touches. The advisory board provides more long-term, strategic advice to MDISS leadership, while the Leadership Circle (above) is generally most concerned with activities that fall into a more tactical twelve-month time window.
Billy Rios,
CEO, Whitescope
"Patient encounters with connected -- yet poorly secured -- medical devices are increasing exponentially, and nobody really has a handle on the risks we’re facing. We’ve got to integrate best practices from cybersecurity, public health and clinical engineering disciplines to better understand and mitigate these threats, and the new MDISS network of WHISTL device testing and data sharing facilities are a huge step in the right direction."